Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) was founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information, visit our webpage.
In this Issue
POCUS for Global Health
Echoes of the Fatu: A Case Report of Rheumatic Heart Disease Detection by Ultrasound in a Samoan Child
By Christopher Snider, MS MA and Melissa Myers, MD
The opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Case
A pediatric patient in rural Samoa presented for routine care when a physician auscultated a new murmur, raising concern for rheumatic heart disease (RHD), which is endemic in the region. Due to the absence of local laboratory diagnostics and limited medical history, compounded by a significant language barrier, the clinical narrative was sparse. The child’s mother reported a febrile illness consistent with streptococcal pharyngitis approximately one month prior, although no confirmatory testing was available.
A POC (Point of Care) Limited Echocardiogram showed moderate mitral regurgitation as well as possible damage to the mitral valve (see Figures 1–6). These findings were concerning for latent RHD based on simplified criteria adapted from the 2023 World Heart Federation (WHF) guidelines. Monthly benzathine penicillin G (BPG) prophylaxis was indicated, but local supply shortages required substitution with daily oral penicillin V. The patient was advised to return in one month for intramuscular prophylaxis and referred to a regional hospital for pediatric cardiology consultation, though the likelihood of follow-up was uncertain.
Video 1: Apical Four Chamber (A4C) with “hockey stick” changes consistent with rheumatic heart disease
Video 2: A4C with moderate mitral valve regurgitation
Video 3: Parasternal long axis with decrease movement of the mitral valve
Discussion
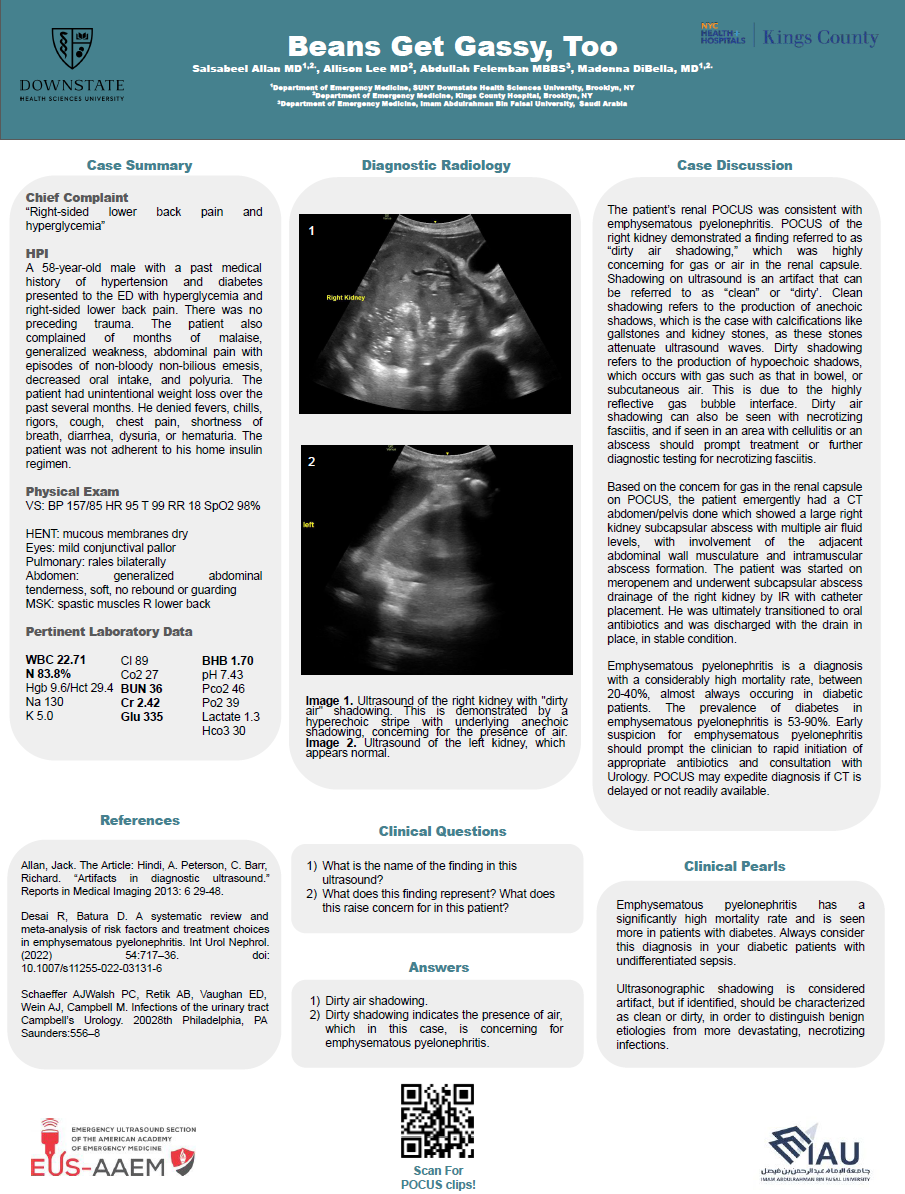
This case highlights the crucial role of focused cardiac ultrasound in the early detection of RHD in low-resource settings. While auscultation remains a standard method for initial screening, it has low sensitivity, particularly for subclinical disease.4 In contrast, the POC Limited Echocardiogram using simplified criteria—such as a left atrioventricular (mitral) regurgitant jet length ≥2.0 cm—has demonstrated diagnostic sensitivity of 77–84% and specificity of up to 92%.¹ ²
The WHF’s updated diagnostic criteria emphasize the detection of morphological valve changes and pathological regurgitation, which are visible on basic imaging modalities.³ In highly endemic regions such as Samoa, rapid school-based screening studies have found a high burden of advanced RHD, often in asymptomatic children.⁴
Delayed diagnosis of RHD can lead to preventable complications, including heart failure, stroke, and premature death.³ Although early detection is vital, long-term management is equally essential. The WHO and WHF recommend regular secondary prophylaxis, typically monthly intramuscular BPG, for at least 10 years following diagnosis.³ However, geographic isolation, penicillin shortages, and unreliable healthcare access make adherence extremely difficult for many families in the South Pacific. In this case, while initial therapy was started, barriers to follow-up and sustainable care persist.
Patient and caregiver education is a crucial pillar of RHD control, particularly in regions where linguistic, economic, or cultural barriers to care exist. Ensuring that families understand the importance of long-term prophylaxis, even in the absence of symptoms, may significantly improve adherence and reduce the risk of recurrence. The revised Jones Criteria highlight the importance of education and adherence in high-risk populations, especially where echocardiographic findings may identify subclinical disease.⁵ These guidelines emphasize that the successful implementation of diagnostic advancements must be paired with culturally informed education to improve health outcomes.³ ⁵
Focused cardiac ultrasound offers a practical solution in such environments. Devices are increasingly affordable and training models have shown that even briefly trained health workers can achieve diagnostic accuracy rivaling that of experienced cardiologists.¹ ² This decentralization of diagnostic capability could radically shift the epidemiologic trajectory of RHD if paired with improved prophylaxis delivery systems and community engagement.
Conclusion
Focused cardiac ultrasound enabled on-site diagnosis of probable rheumatic heart disease in a Samoan child without access to traditional diagnostic pathways. This case illustrates the potential of FoCUS to bridge gaps in early detection, especially where auscultation and clinical history are insufficient. Strategic deployment of handheld echocardiography, guided by simplified WHF criteria, may offer a scalable approach to reducing the global burden of RHD, provided it is integrated with reliable prophylaxis and longitudinal care infrastructure.
References
- Mirabel M, Bacquelin R, Tafflet M, et al. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ: Cardiovascular Imaging. 2015;8(1):e002324. doi:10.1161/CIRCIMAGING.114.002324
- Engelman D, Kado JH, Reményi B, et al. Focused cardiac ultrasound screening for rheumatic heart disease by briefly trained health workers: a study of diagnostic accuracy. The Lancet Global Health. 2016;4(6):e386-e394. doi:10.1016/S2214-109X(16)30065-1
- Rwebembera J, Marangou J, Mwita JC, et al. 2023 World Heart Federation guidelines for the echocardiographic diagnosis of rheumatic heart disease. Nat Rev Cardiol. 2024;21(4):250-263. doi:10.1038/s41569-023-00940-9
- Allen M, Allen J, Naseri T, Gardner R, Tolley D, Allen L. A rapid echocardiographic screening protocol for rheumatic heart disease in Samoa: a high prevalence of advanced disease. Cardiol Young. 2017;27(8):1599-1605. doi:10.1017/S1047951117000907
- Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the Diagnosis of Acute Rheumatic Fever in the Era of Doppler Echocardiography: A Scientific Statement From the American Heart Association. Circulation. 2015;131(20):1806-1818. doi:10.1161/CIR.0000000000000205
POCUS Saves
Clot in Transit: A Rapidly Fatal Threat Identified by POCUS
By Hanna Hussein, MD and Lauren Rosenblatt, MD
Case
A 51-year-old female with a past medical history of metastatic ovarian cancer and hypertension was brought to the emergency department as a rapid response from the cancer infusion clinic due to severe respiratory distress and tachypnea. The patient was placed on a non-rebreather mask. Multiple attempts to obtain an initial blood pressure were unsuccessful.
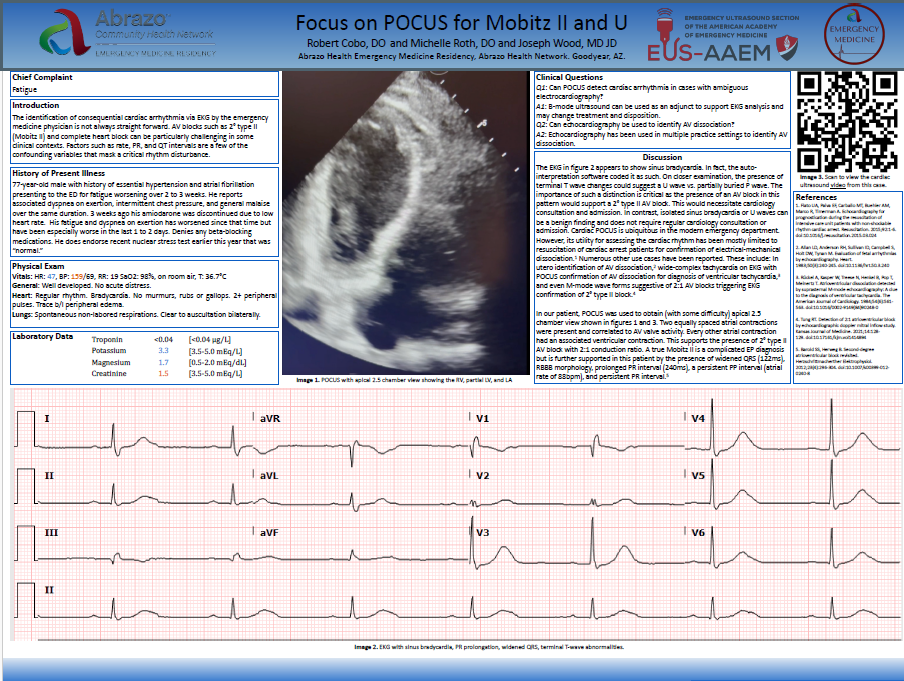
Bedside point-of-care ultrasound (POCUS) was performed, revealing a large clot in transit and a D-sign (Figures 1 and 2).
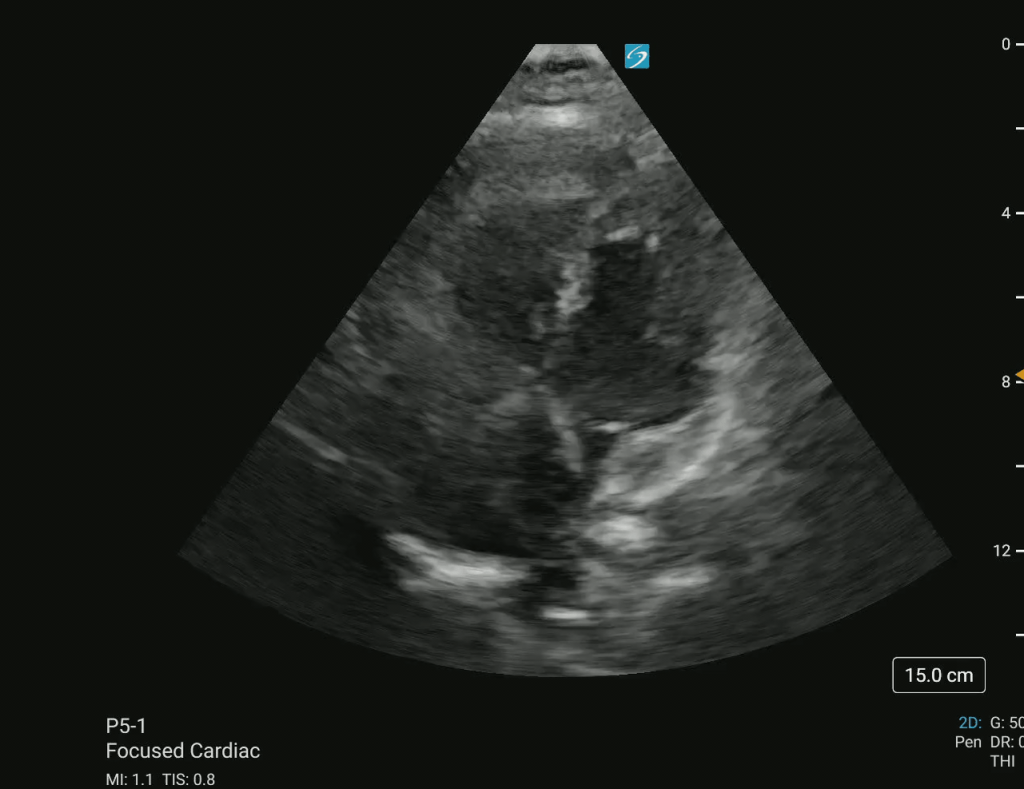
Figure 1 – Apical four-chamber (A4CH) view showing a massive clot in transit in the right atrium (RA) and right ventricle (RV).
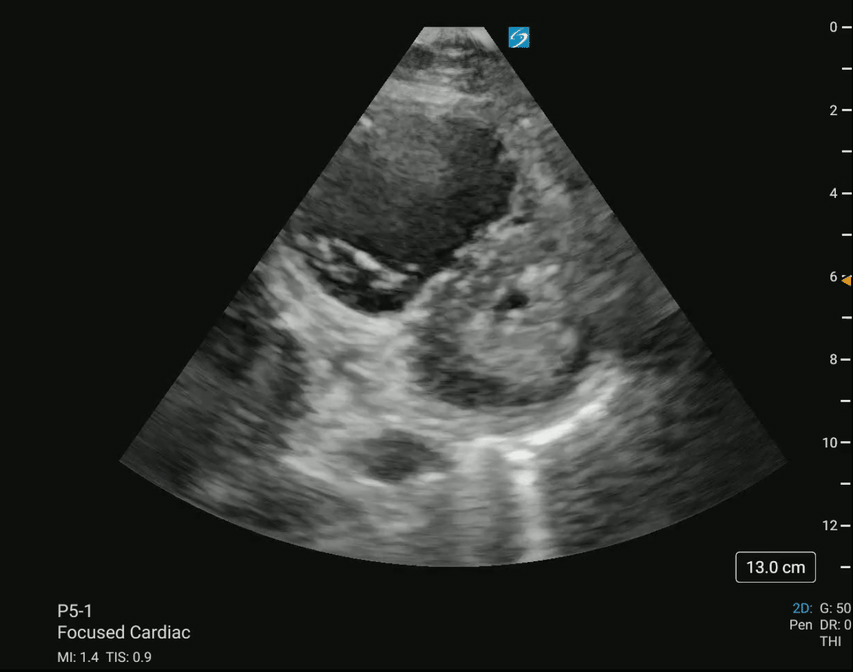
Figure 2 – Parasternal short-axis (PSAX) view demonstrating a D-sign, indicative of right ventricular strain.
On initial examination, the patient appeared diaphoretic, ill, and toxic. Cardiovascular examination revealed tachycardia with a regular rhythm and palpable femoral pulses; however, radial and dorsalis pedis pulses were absent. Pulmonary examination showed severe respiratory distress, tachypnea, and tripoding. Capillary refill was delayed at more than three seconds. Initially, the patient was alert and able to state her name while moving all four extremities, though further examination was limited due to the acuity of her condition.
The patient was started on norepinephrine for blood pressure support due to suspected massive pulmonary embolism (PE) identified on POCUS. However, she subsequently became bradycardic, with heart rates in the 40s, and lost pulses. Cardiopulmonary resuscitation was initiated, and the initial rhythm was pulseless electrical activity (PEA). The patient received epinephrine and was given Tenecteplase via an emergently placed right femoral central venous catheter. She was also intubated for airway protection. ROSC was achieved approximately 15 minutes later. The patient was subsequently taken for thrombectomy and admitted to the intensive care unit.
Discussion
The presence of a clot in transit, as visualized on POCUS, is a rare but life-threatening finding in patients with suspected PE. Right heart thrombi (RHT) are strongly associated with massive PE and an increased risk of hemodynamic collapse and mortality, with reported mortality rates as high as 45% even with treatment.2,3 Early identification of RHT is crucial, as it signals an impending or ongoing embolic event that can rapidly lead to cardiovascular collapse, as was seen in this case. POCUS provided rapid bedside identification of the clot, which facilitated prompt escalation of care, including hemodynamic support, thrombolysis, and advanced resuscitation efforts.
Systemic thrombolysis remains the first-line therapy for massive PE, particularly in cases of cardiac arrest, as it improves survival rates and increases the likelihood of achieving ROSC. Recent studies have demonstrated that thrombolytic therapy during PE-induced cardiac arrest can lead to improved outcomes, particularly when administered early in the resuscitation process.4 In this case, Tenecteplase was administered via an emergently placed central venous catheter during CPR, leading to ROSC shortly after. While systemic thrombolysis is often the initial treatment, other interventions such as catheter-directed thrombolysis or surgical embolectomy should be considered in patients with contraindications to fibrinolysis or persistent hemodynamic instability despite treatment.3
The role of POCUS in the assessment of critically ill patients with undifferentiated shock or respiratory distress continues to expand, particularly in emergency and critical care settings. In patients with suspected PE, echocardiographic findings such as right ventricular strain, McConnell’s sign, and direct visualization of a clot in transit can provide immediate diagnostic information and help guide therapeutic decisions.1 The advantage of POCUS is its ability to provide real-time information at the bedside, particularly in unstable patients who cannot be safely transported for CT pulmonary angiography. In this case, the use of bedside ultrasound allowed for rapid diagnosis and initiation of life-saving interventions, reinforcing its value as an essential tool in the management of PE-related hemodynamic instability.
Despite aggressive resuscitation and early thrombolysis, patients with massive PE and RHT remain at high risk for adverse outcomes, including recurrent embolic events and post-arrest complications. Multidisciplinary collaboration involving emergency medicine, critical care, and interventional radiology teams is critical for optimizing management in these cases.
Conclusion
This case highlights the critical role of point-of-care ultrasound in the rapid diagnosis and management of life-threatening pulmonary embolism. The identification of a clot in transit in the right atrium and ventricle provided immediate confirmation of PE, guiding the decision for thrombolysis during cardiac arrest. The successful ROSC following Tenecteplase administration underscores the potential life-saving benefits of timely fibrinolytic therapy in massive PE. This case reinforces the importance of bedside ultrasound in emergency and critical care settings, emphasizing its utility in the rapid assessment of hemodynamically unstable patients.
References
- Benser A, Carlson C, Casper E, Ignatowski D, Jain R, Wessly P. Many roads traveled: Sonographer insights on clots in transit—an echocardiography case series. CASE. Published online March 2025. doi:10.1016/j.case.2025.01.004
- Kabrhel C, Rosovsky R, Garvey S. Special considerations in Pulmonary embolism. Critical Care Clinics. 2020;36(3):531-546. doi:10.1016/j.ccc.2020.02.008
- Khosla A, Mojibian H, Assi R, Tantawy H, Singh I, Pollak J. Right heart thrombi (RHT) and clot in transit with concomitant PE management: Approach and considerations. Pulmonary Circulation. 2022; 12:e12080. Doi:10.1002/pul2.12080
- Rolon L, Quevedo S, Martinez Nunez A, et al. A clot-in-transit: An imminent threat. CHEST. 2023;164(4). doi:10.1016/j.chest.2023.07.3954
Unveiling the Hidden Obstruction: A POCUS Revelation in an Elderly Patient with Dyspnea
By Carmen Reyes-Jimenez, Stephanie Marrero-Borrero, Michelle Surillo-Gonzalez, Karyna Soto Rosa, and Nicole Ocasio Martínez
Introduction
Hypertrophic obstructive cardiomyopathy (HOCM) is a well-recognized cause of sudden cardiac death in young individuals, but it’s late-onset presentation in elderly patients is rare and often misdiagnosed as heart failure (HF), ischemic heart disease, or aortic stenosis. In the emergency department (ED), failure to recognize HOCM can lead to inappropriate treatment, worsening hemodynamic instability, and increased mortality. This case highlights the critical role of point-of-care ultrasound (POCUS) in diagnosing HOCM for the first time in an elderly female of 74-year-old who presented with acute shortness of breath (SOB), new-onset atrial fibrillation (A-Fib), and non-ST elevation myocardial infarction (NSTEMI).
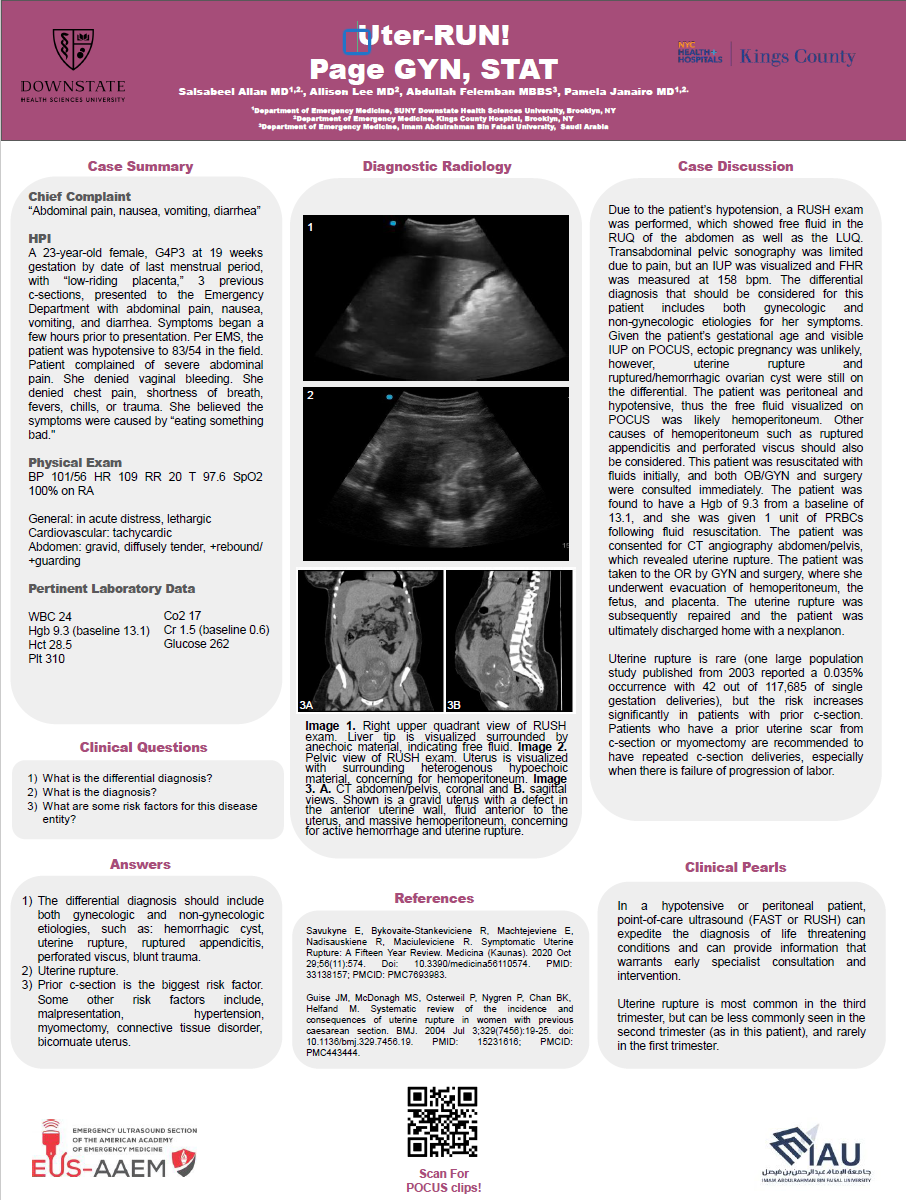
HCM has a prevalence of 1 in 500 and should be suspected with septal thickness ≥15mm or with other left ventricular or apical hypertrophy. M-mode imaging may demonstrate systolic anterior mitral valve motion, which is specific for HCM.1 The left ventricular outflow tract (LVOT) gradient is another way bedside ultrasound can be utilized in the diagnosis of obstructive HCM. This is performed by placing the continuous wave Doppler beam over the LVOT in an apical 5-chamber view. A gradient > 30 mmHg (2.7 m/s) is defined as intraventricular obstruction. Asymmetric septal hypertrophy. Interventricular septum/posterior wall thickness ratio of: >1.3 in normotensive patients or >1.5 in hypertensive patients is another diagnostic criteria.2 In our case bedside ultrasound revealed basal anteroseptal wall hypertrophy, systolic anterior motion (SAM) of the mitral valve normal—and an apical four-chamber view using color flow displaying dynamic left ventricular outflow tract (LVOT) obstruction, confirming the diagnosis of HOCM. Standard heart failure treatments, including diuretics, vasodilators, and inotropes, can exacerbate LVOT obstruction, leading to cardiovascular collapse. This case underscores the importance of POCUS in differentiating HOCM from other causes of dyspnea and heart failure in elderly patients. Emergency physicians must develop proficiency in bedside echocardiography to rapidly identify septal hypertrophy, SAM, and LVOT obstruction, ensuring timely and appropriate management to prevent life- threatening complications.
Case Presentation
A 74-year-old female with a past medical history of arterial hypertension, non-insulin- dependent diabetes mellitus type 2, hyperlipidemia, and congenital hearing loss was transferred to the ED due to progressive SOB and dry cough for three days, associated with chest pain and subjective fever as reported by a family member. She was initially managed at an outside hospital, where she was diagnosed with NSTEMI and new-onset atrial fibrillation (A-fib). Treatment at the referring facility included ceftriaxone, diltiazem, and respiratory nebulization therapy.
Upon arrival at our ED, her vitals T: 36.5, HR: 121 bpm, BP: 155/99, RR: 20, SatO2: 99% on room air but remained dyspneic. The patient’s electrocardiogram showed atrial fibrillations. No active chest pain was reported at that time of examination. Given her history of NSTEMI and A-fib, an urgent POCUS was performed to assess cardiac function.
Images & Findings
Video 1: Parasternal long-axis view (PLAX): Showed asymmetric septal hypertrophy
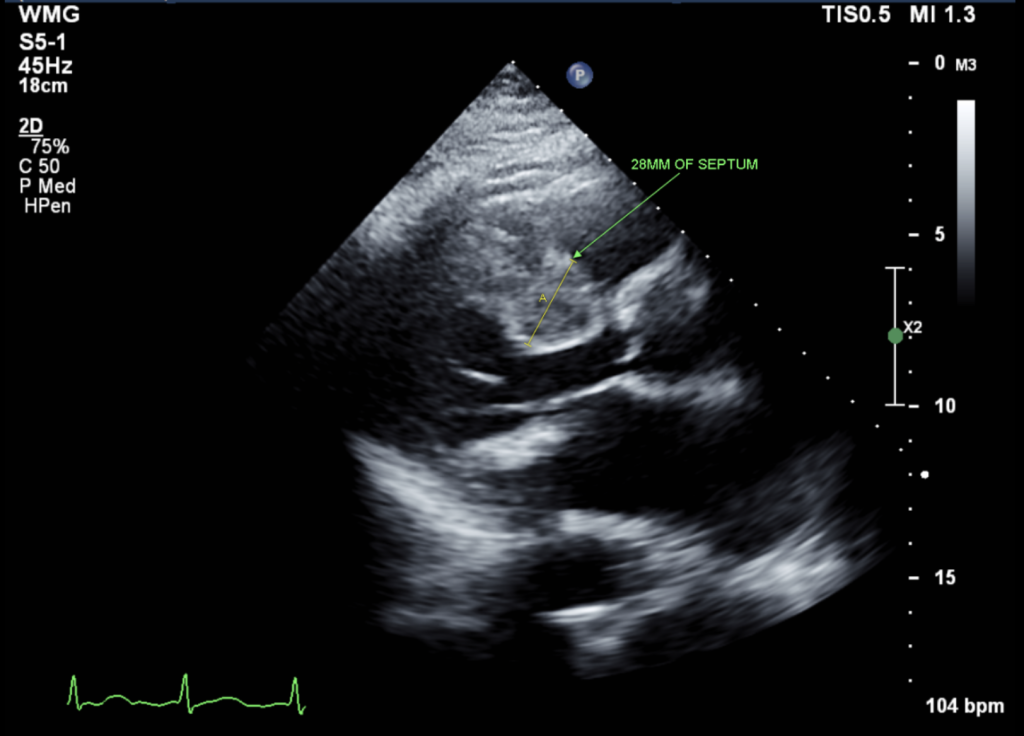
Figure 1: Parasternal long-axis view (PLAX): Showed asymmetric septal hypertrophy (28 mm)
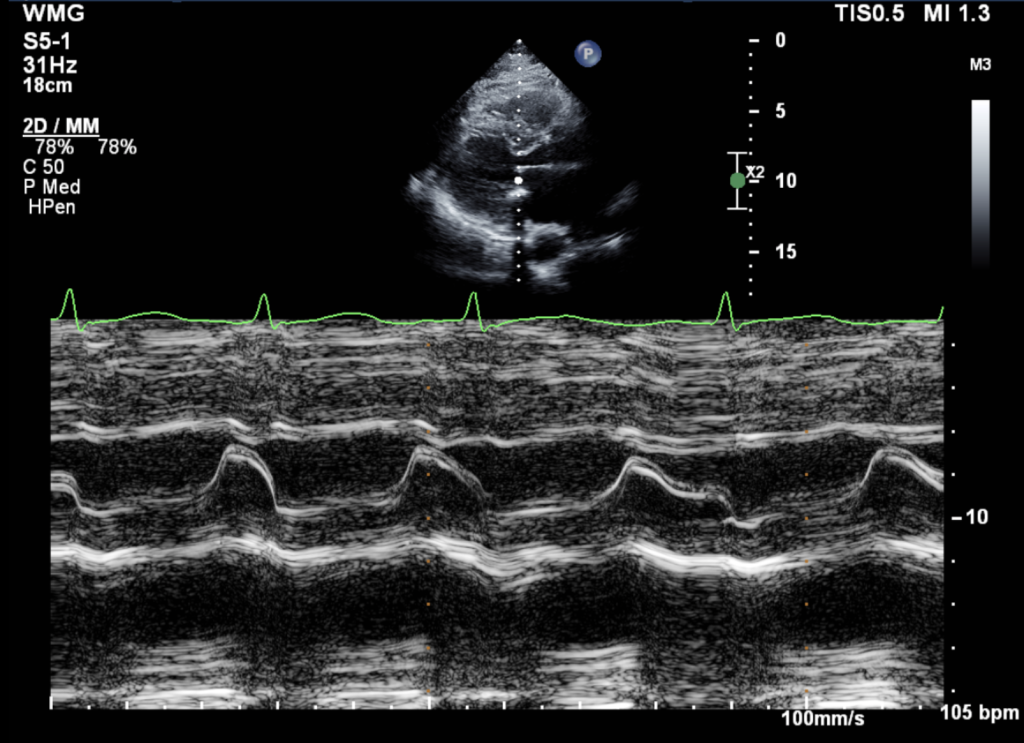
Figure 2: M-mode through the mitral valve: No systolic anterior motion (SAM) of the mitral leaflet was present.
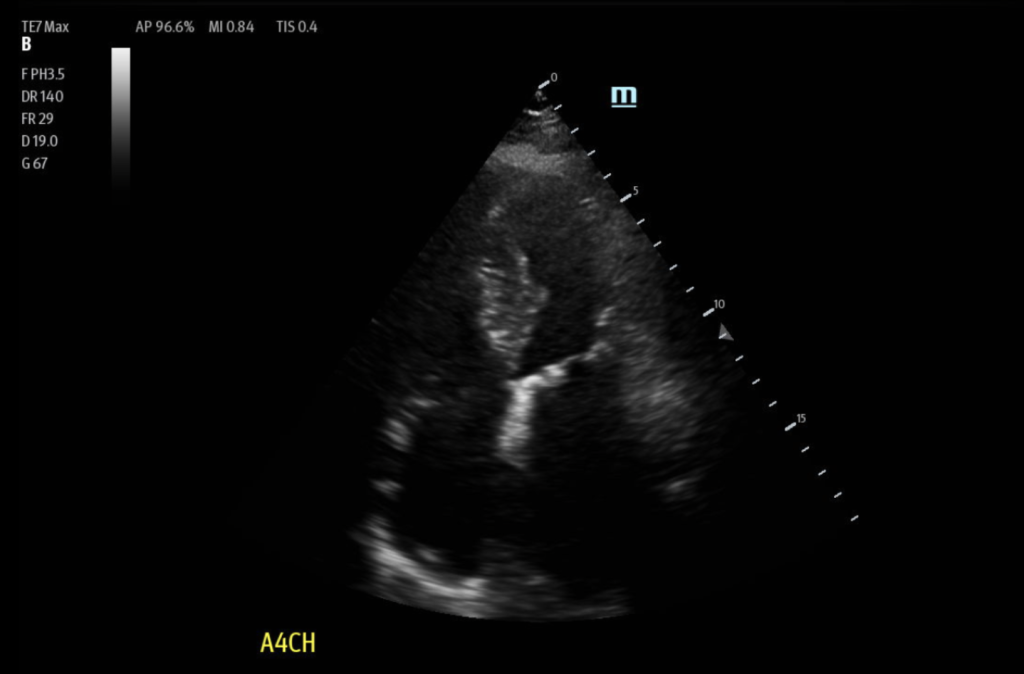
Figure 3: Apical 4 Chamber view (A4C): Showed asymmetric septal hypertrophy
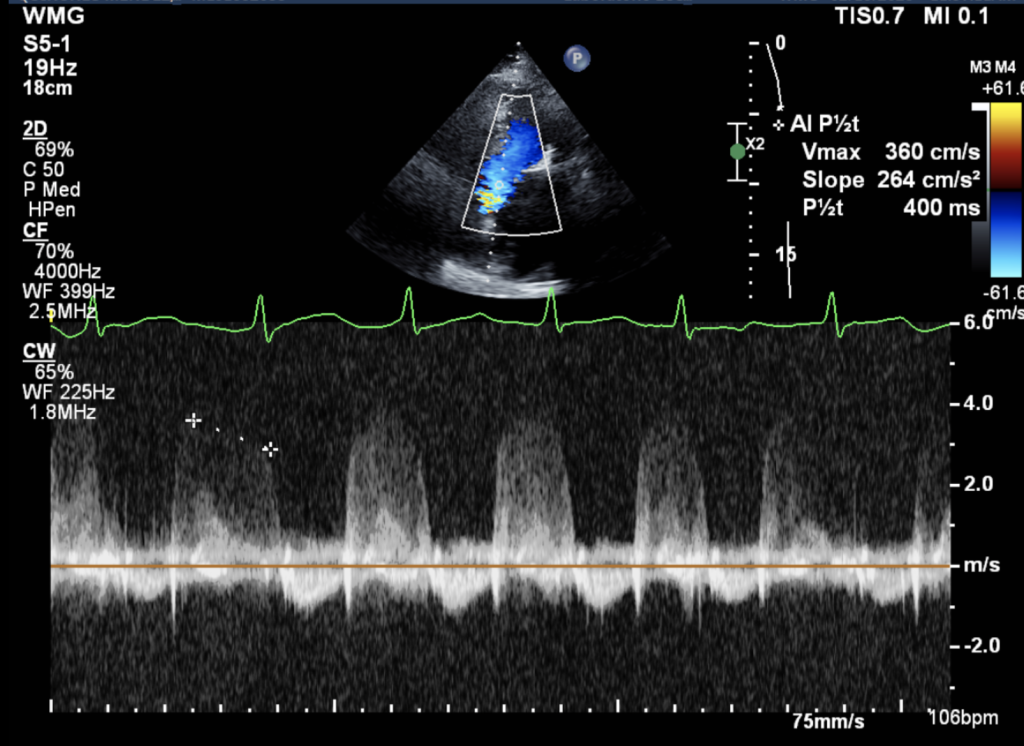
Figure 4: Color Doppler: Identified turbulent flow across the LVOT and Continuous wave Doppler: Revealed increased peak LVOT velocity (>3.0 m/s), consistent with dynamic obstruction.
Cardiology team TEE demonstrated mild concentric left ventricular hypertrophy with proximal septal thickening, severe biatrial dilation, and high left atrial pressure (LAP Grade 2) and a preserved ejection fraction (50-55%) with severe diastolic dysfunction.
Discussion
HOCM has an annual mortality rate of about 0.5% predisposes patients to arrhythmias, particularly atrial fibrillation, sudden cardiac death, cardiac ischemia, and systolic heart failure.3 LVOT obstruction due to HOCM is often misdiagnosed as heart failure or ischemia, delaying proper management. There’s no available large meta analysis data in order to determine sensitivity or specificity of the diagnosis of HOCM with POCUS in elderly patients.
In elderly patients presenting with dyspnea and atrial fibrillation de novo, although rare, HOCM should be considered in the differential diagnosis alongside HF, significant coronary artery disease, aortic stenosis, and pulmonary embolism. Unlike younger patients who often present with exertional syncope or palpitations, elderly patients with HOCM frequently develop dyspnea, angina, and arrhythmias, which can lead to misdiagnosis. In this case, POCUS was instrumental in differentiating HOCM from other causes of heart failure and ischemia, allowing for appropriate management. Standard heart failure treatments, such as diuretics and vasodilators, reduce preload and afterload, exacerbating LVOT obstruction and leading to hemodynamic deterioration. Similarly, inotropes such as dobutamine, which increase myocardial contractility, worsen obstruction and can precipitate cardiogenic collapse. Instead, optimal management includes beta-blockers or non-dihydropyridine calcium channel blockers such as metoprolol or verapamil, which reduce LVOT obstruction by decreasing heart rate and myocardial contractility. In the ED, the management used consisted of diltiazem for rate control. Anticoagulation is essential in patients with HOCM and atrial fibrillation to mitigate the risk of thromboembolic events. Careful volume resuscitation is necessary to maintain preload and left ventricular filling, while cardiology consultation is warranted for consideration of septal reduction therapy, such as alcohol septal ablation or myectomy, in select patients.
Conclusion
This case highlights the role of POCUS in the ED for early recognition of LVOT obstruction in adult or elderly patients who were not previously diagnosed with HOCM. Most patients are misdiagnosed as having HF or ischemia in the ED, leading to inappropriate treatment and increased morbidity. Cardiac ultrasound is a core POCUS modality that acute care providers must master to rapidly identify septal hypertrophy, systolic anterior motion (SAM), and dynamic LVOT obstruction at the bedside. POCUS is highly sensitive and specific for cardiac pathologies, enabling ED physicians to make real-time diagnoses, guide management, and refer patients for appropriate cardiology follow-up. Detecting HOCM earlier could prevent further deterioration of cardiac dysfunction. Integrating bedside echocardiography into routine cardiac assessment improves diagnostic accuracy, optimizes treatment, and prevents life-threatening hemodynamic collapse in patients with late-onset HOCM.
References
- Long MT, Lam S. Point-of-Care Ultrasound to Evaluate a Teenager with Presyncope. West J Emerg Med. 2016 Mar;17(2):195. doi: 10.5811/westjem.2015.12.28922. Epub 2016 Mar 2. PMID: 26973751; PMCID: PMC4786245.
- Fischer, K., & Taylor, L. (2023, January 20). Motion of the HOCean: A clinical case of hypertrophic obstructive cardiomyopathy (HOCM). https://www.acep.org/emultrasound/newsroom/jan2023/motion-of-the-hocean-a-clinical-case-of-hypertrophic-obstructive-cardiomyopathy
- Stoll CM, Carr M, Naraghi L. Hypertrophic Cardiomyopathy Diagnosed on Point-of-Care Echocardiogram in an Elderly Patient With Syncope. Cureus. 2021 Aug 8;13(8):e17008. doi: 10.7759/cureus.17008. PMID: 34540409; PMCID: PMC8423594.
POCUS Procedures
Point of Pain – Ultrasound Guided Hip Arthrocentesis in the Emergency Department
By Brianna Mendiola, MD and Michael Rosselli, MD
Introduction
Hip joint effusions can be more difficult to identify and aspirate when compared to effusions in other joints that are commonly managed by emergency physicians, though it’s an established procedure in other fields such as orthopedic surgery and interventional radiology. An ultrasound is considered positive for an effusion if the effusion is 5mm or greater as measured from the anterior surface of the femoral neck to the posterior surface of the iliopsoas muscle or if there is a difference of 2mm or greater between the symptomatic and contralateral hip. Hip arthrocentesis can help confirm diagnosis and guide treatment of various hip conditions such as septic arthritis, transient synovitis, hemarthrosis, gout, pseudogout, etc. Performing a hip arthrocentesis in the emergency department can be a crucial step in the timely management of joint disorders, essential diagnostic information, and most importantly providing rapid relief of symptoms by simple aspiration of an effusion.
Indications
There are several indications for performing hip arthrocentesis in an emergency setting:
- Suspected septic arthritis: To diagnose or rule out an infection within the joint.
- Gout or pseudogout: To identify crystal-induced arthritis by analyzing the synovial fluid for crystals.
- Unexplained joint effusion: To determine the cause of excess fluid accumulation in the joint.
- Hemarthrosis: To assess bleeding within the joint, often due to trauma or underlying coagulopathy.
- Pain relief: To relieve pressure and discomfort caused by joint effusion.
Contraindications
While hip arthrocentesis is generally safe, some contraindications to be aware of:
- Overlying skin infection: To avoid introducing pathogens into the joint space.
- Bleeding disorders: Patients with coagulopathies may be at higher risk of bleeding complications.
- Lack of anatomical landmarks: Difficulty in locating the joint space may increase the risk of complications.
Ultrasound Procedure
- Ensures precision of procedure because you are able to visualize joint space
Steps:
- Patient laying supine, sterile gloves, antiseptic solution, topical and local anesthetic, needle (18-21 gauge), syringe, and sterile dressing.
- Locate femoral vessels with probe marker pointed to the right of the patient and locate humeral head deep to these structures
- Rotate probe, with marker pointing toward the umbilicus and you should be able to identify the following structures: femoral head, femoral neck, anterior synovial recess
- In-plane approach from the side opposite of probe marker with 20 gauge spinal needle attached to 10/20cc syringe. Having some anesthetic already in the syringe to inject during the procedure before reaching the anterior synovial recess will help the patient tolerate the procedure and help provide pain relief
- Once in the synovial recess, aspirate the effusion
- Withdraw needle and apply sterile dressing to the puncture site
- Fluid sent for further analysis
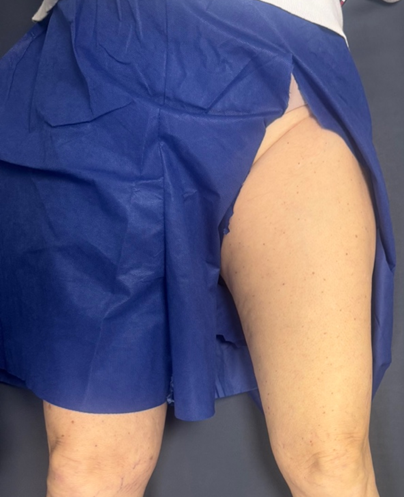
Figure 1: Position and patient exposure
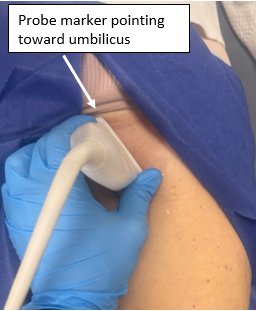
Figure 2: Probe positioning
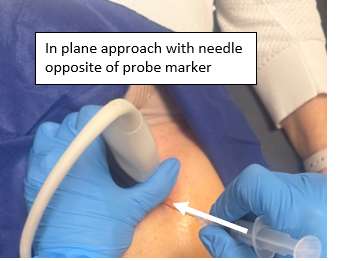
Figure 3: Needle positioning
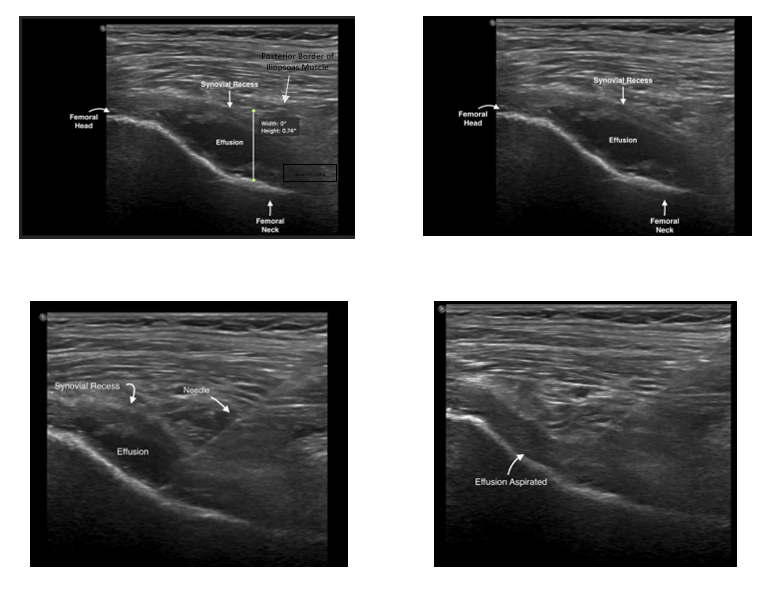
Figure 4
Post-procedure Analysis
Synovial fluid analysis is very important in diagnosing joint conditions:
- Gram stain and culture: To identify bacterial organisms in cases of suspected septic arthritis.
- Crystal analysis: To detect urate or calcium pyrophosphate crystals in cases of gout or pseudogout.
- Cell count and differential: To assess inflammation by measuring white blood cell count.
- Glucose and protein levels: To provide additional information on the nature of the joint effusion. (can take this bulletin off)
Conclusion
Hip arthrocentesis under ultrasound guidance in the emergency department is a valuable procedure that aids in the rapid diagnosis and management of various hip complaints such as septic arthritis, transient synovitis, hemarthrosis, gout, pseudogout, etc. With the use of ultrasound, it can be done with much more ease and decreased margin of error. By following proper techniques and precautions, healthcare providers can minimize risks and ensure optimal outcomes for their patients. This procedure not only provides immediate relief from a joint effusion, but also expedites critical diagnostic information that guides further treatment decisions.
References
Chen KC, Lin AC, Chong CF, Wang TL. An overview of point-of-care ultrasound for soft tissue and musculoskeletal applications in the emergency department. J Intensive Care. 2016 Aug 15;4:55. doi: 10.1186/s40560-016-0173-0. PMID: 27529031; PMCID: PMC4983782.
Freeman K, Dewitz A, Baker WE. Ultrasound-guided hip arthrocentesis in the ED. Am J Emerg Med. 2007 Jan;25(1):80-6. doi: 10.1016/j.ajem.2006.08.002. PMID: 17157689.
Roberts, W. N., & Hayes, C. W. (2024, December 10). Joint aspiration and injection in adults: Indications and technique. In: UpToDate, Connor RF (Ed), Wolters Kluwer. (Accessed on February 7, 2025)
Thom, C., Ahmed, A., Kongkatong, M., & Moak, J. (2020). Point-of-care hip ultrasound leads to expedited results in emergency department patients with suspected septic arthritis. Journal of the American College of Emergency Physicians open, 1(4), 512–520. https://doi.org/10.1002/emp2.12167
AAEM25 Photo Contest Winners
1st Place:
2nd Place:
3rd Place: